Caritas Coimbra is a supporter of the European Commission Breast Cancer Initiative (ECIBC)
Caritas Coimbra is a supporter of the European Commission Breast Cancer Initiative (ECIBC)

ECIBC: Quality and Equality in Breast Cancer Care
What is ECIBC?
The European Commission Initiative on Breast Cancer (ECIBC) is a person-centred initiative to improve and harmonise breast cancer care in Europe.
ECIBC is developing the most up-to-date evidence-based recommendations on breast cancer screening and diagnosis, together with a platform of high-quality, evidence-based guidelines covering the whole care pathway. These serve as a basis for developing a quality assurance scheme applicable by breast cancer services.
ECIBC stems from the will of the European institutions and Member States. Since its launch in 2012, the Commission’s Directorate General for Health and Food Safety and the Joint Research Centre have been closely cooperating towards its final implementation.
ECIBC is a tangible demonstration of what Europe can achieve working in synergy with Member States, the scientific community, civil society, and patient organisations.
Why ECIBC?
Breast cancer is the most common cancer in Europe and the most common cause of cancer death in women.
Although the incidence of breast cancer has increased at a different rate across European countries, mortality has decreased since the mid-1990s, but not everywhere in Europe. The existence of significant disparities in breast cancer mortality patterns across the EU, suggests possible inequalities in healthcare provided in individual MS. ECIBC addresses these inequalities with the aim of reducing the burden of cancer at European level.
What are the ECIBC objectives?
ECIBC has the ultimate goal of establishing the European quality assurance scheme for breast cancer services, addressing all care processes including screening, diagnosis, treatment, rehabilitation, survivorship care, and palliative care[1].
The European QA scheme sets the requirements and indicators in order to guarantee that breast cancer care is effective, safe, and person-centred.
Within the European QA scheme, the European Breast Guidelines cover the screening and diagnosis, while the post-diagnosis part of the cancer pathway is covered by the Guidelines Platform.
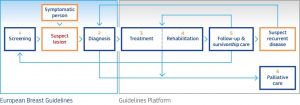
Finally, ECIBC aims to propose a European training template on digital breast screening for radiologists and radiographers to support the competences of involved professionals.
Which are the benefits for women and patients?
ECIBC empowers women and patients. Once the breast cancer services have implemented the European QA scheme, the website will map the accredited/certified centres, enabling women and patients to make an informed decision on where to go for care.
The European QA scheme focuses on women’s needs and ensures that the screening and care pathway are fully covered.
The ECIBC website hosts the recommendations that are progressively released, in different versions made bespoke for the needs of professionals, patients and individuals, and the policy makers. The recommendations will be available in all EU official languages.
How can I get involved?
ECIBC applies an inclusive and bottom-up approach. ECIBC is supported by two working groups composed of medical doctors and other healthcare professionals, researchers, and patients selected through a transparent process to join the project on a voluntary basis after a public call for expression of interest.
Everyone is welcome to participate to the calls for feedback on specific documents, as well as for general expression of interest, launched on line at crucial stages of the development.
Feedback is essential for ECIBC, now and when the European QA scheme will be established: users’ feedback nourishes the lifecycle of the scheme, helping with adjustment and continuous improvements.
Timeline
- European Breast Guidelines – ongoing and progressive web publication
- Guidelines Platform – 2018
- Digital breast screening training template – 2018
- Piloting of the European QA Scheme starting in 2018, and final release in 2019
LATEST ECIBC RECOMMENDATIONS ON BREAST CANCER SCREENING AND DIAGNOSIS
Want to know more?
Please visit the ECIBC website at: ecibc.jrc.ec.europa.eu
Contacts
European Commission • Joint Research Centre (JRC)
Health, Consumers and Reference Materials
Health in Society
Via E. Fermi 2749 • 21027 Ispra (VA) • Italy
Tel.: +39 0332 78 9861
jrc-cancer-policy-support@ec.europa.eu
[1] Embedded in the European accreditation legal framework.

Leave a Reply
You must be logged in to post a comment.